

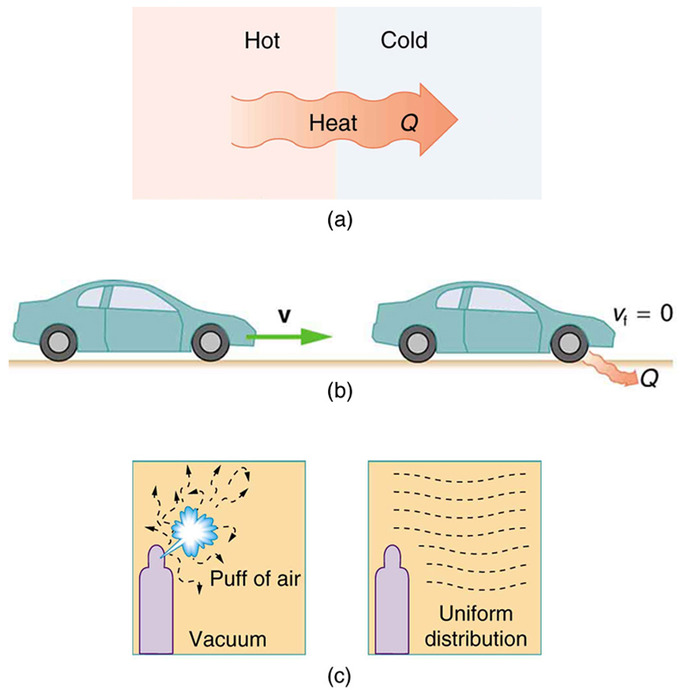
Kelvin-Planck Statement of the Second Law of Thermodynamics.

Importance of Second Law of Thermodynamics.Entropy and Second Law of Thermodynamics.Study Newton’s Second Law Of Thermodynamics Here However, before proceeding with the explanation for the second law of thermodynamics and what it represents, it is important to understand the Entropy of a system. Understanding the Entropy of a system and how it is related to the spontaneity of a system is what the second law of thermodynamics explains in detail. The spontaneity or the disturbance in the system, established by the term ‘entropy’, is taken into account in the second law of thermodynamics. The law could not explain the spontaneity or the feasibility of the reaction. The first law of thermodynamics establishes a relation between the heat changes and work done by a system, but it doesn’t discuss the direction of the flow of heat. Or “The total mass and energy of an isolated system remain unchanged.” Entropy and Second Law of Thermodynamics: The first law of thermodynamics states that “Energy can neither be created nor destroyed it can only be transformed from one form to another”


 0 kommentar(er)
0 kommentar(er)
